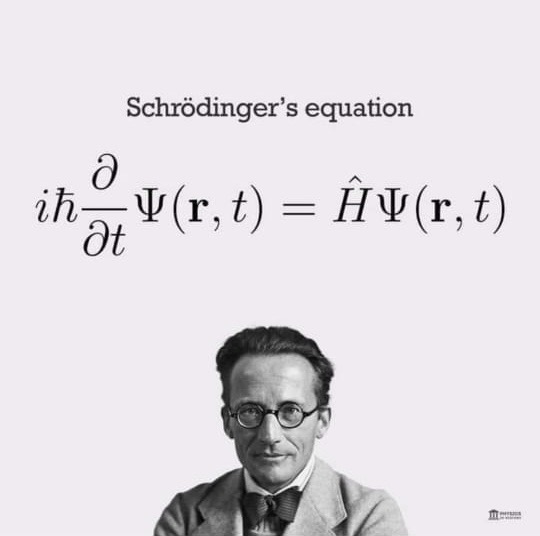If as a young graduate you plan to be a scientist, start not just enjoying the beauty of Nature which is easy but more importantly the beauty and serenity of differential equations in particular the Schrödinger Wave Equation.
Modern physics started in 1905 with Einstein famous paper on Special theory of relativity
The year often considered as the start of modern physics is 1905. This is because in that year, Albert Einstein published his special theory of relativity, which revolutionized our understanding of space, time, and energy. Additionally, in the same year, he also published papers on the photoelectric effect and Brownian motion, further contributing to the foundation of modern physics.
Max Planck’s black body radiation formula introduced several key features:
Planck proposed that energy is quantized, meaning it can only exist in discrete units or “packets” called quanta. This was a departure from classical physics which assumed continuous energy.
The formula relates the energy of a photon (E) to its frequency (ν) through the equation \(E = hν\), where \(h\) is Planck’s constant.
The intensity of radiation emitted by a black body at a given frequency increases exponentially with frequency, which accurately described experimental observations.
Planck’s formula successfully resolved the ultraviolet catastrophe, a problem in classical physics which predicted infinite energy for high-frequency radiation.
At large wavelengths (low frequencies), the formula converges with classical physics, which was essential in ensuring Planck’s theory matched known behavior in the limit of low frequencies.
These features marked a significant departure from classical physics and laid the groundwork for the development of quantum theory.
Niels Bohr successfully used Planck’s quantization and developed his own model of the atom, known as the Bohr model, to explain the stability of atoms and line spectra.
Here’s how he did it:
Bohr proposed that electrons orbiting the nucleus could only occupy certain discrete energy levels or orbits. This was in line with Planck’s idea of quantized energy.
Bohr combined Planck’s idea with the concept of angular momentum quantization. He postulated that an electron’s angular momentum is quantized and is an integer multiple of \(h/2π\), where \(h\) is Planck’s constant.
Bohr argued that in these quantized orbits, the centripetal force required to keep the electron in its orbit was provided by the electrostatic attraction between the positively charged nucleus and the negatively charged electron.
According to Bohr, when an electron transitions between these quantized orbits, it emits or absorbs energy in the form of electromagnetic radiation. The energy of these photons is related to the energy difference between the initial and final orbits.
Bohr’s model successfully explained line spectra, which are the discrete lines observed in the emission or absorption spectrum of atoms. These lines correspond to specific energy transitions of electrons between quantized orbits.
The Bohr model also addressed the stability of electrons in their orbits. Electrons would only radiate energy when transitioning between orbits, leading to stable orbits and preventing the electron from spiraling into the nucleus, as classical physics had predicted.
Bohr’s model was a major breakthrough in understanding atomic structure, and it laid the foundation for further developments in quantum mechanics. It was later expanded upon and refined by quantum theory, but Bohr’s contributions were crucial in the early stages of this revolution in physics.
Louis de Broglie proposed the concept of wave-particle duality in 1924, suggesting that particles, like electrons, could exhibit both wave-like and particle-like behavior. His idea was based on the following intuitive basis:
De Broglie was inspired by the already established wave-particle duality of light. Light was known to exhibit both wave-like properties (interference, diffraction) and particle-like properties (photoelectric effect).
De Broglie considered Einstein’s famous equation \(E=mc^2\), which demonstrated that energy (E) and mass (m) are interchangeable. This equation hinted at a fundamental connection between particles and waves.
Building on Max Planck’s work, De Broglie recognized that if energy could be quantized for photons (particles of light), it might also be possible for other particles. He suggested that particles like electrons could have wave properties associated with their motion.
De Broglie proposed that any particle with momentum P could be associated with a wavelength lambda given by h/P where h is Planck’s constant. This wavelength is now known as the de Broglie wavelength.
De Broglie’s idea was soon confirmed by experiments, most notably the electron diffraction experiments conducted by Clinton Davisson and Lester Germer in 1927. They observed interference patterns when electrons were diffracted through a crystal lattice, providing strong evidence for the wave-like nature of particles.
De Broglie’s wave-particle duality concept revolutionized our understanding of the behavior of particles at the quantum level. It laid the foundation for the development of quantum mechanics, which has become one of the most successful and fundamental theories in modern physics.
This is the famous wave particle duality of course electron diffraction experiments by Davison Germer conforms this conjuncture.
to be continued